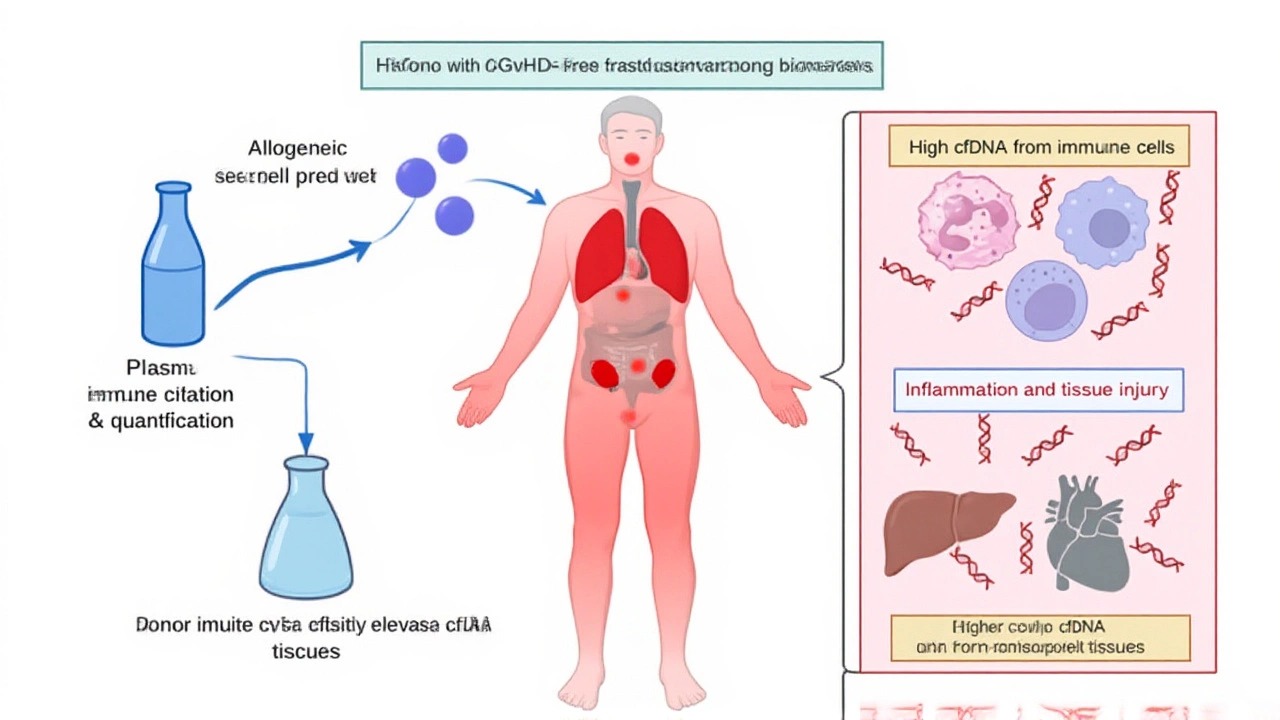
When Avni B, principal investigator and colleagues Neiman D and Shaked ED published their findings in the Journal of Clinical Investigation last October, the transplant world got a new early‑warning system for chronic graft‑versus‑host disease (cGVHD). The study, which tracked 101 recipients of hematopoietic stem cell transplantation (HCT) across several centers, proved that a cocktail of tissue‑specific cell‑free DNA methylation markers could flag organ damage long before doctors see any rash, liver enzyme spike, or lung trouble. In short, a blood test now whispers about hidden tissue turnover when patients feel perfectly fine, opening a window for earlier therapy and potentially better outcomes.
Why cfDNA Methylation Is a Game‑Changer
Cell‑free DNA (cfDNA) floats in the bloodstream as tiny fragments shed by dying cells. What makes these fragments special is that their DNA methylation patterns act like zip codes, pointing to the tissue of origin – liver, skin, lungs, colon, or even specific immune cells. Historically, clinicians have relied on clinical scoring systems or invasive biopsies to gauge cGVHD, both of which can lag behind the actual disease process. The twist is that cfDNA offers a non‑invasive, real‑time snapshot of what’s happening under the skin.
Here’s the thing: the researchers built a "cocktail" of methylation markers tailored to five key organs plus monocyte/macrophage DNA. By measuring the concentration of each fragment in patient plasma, they could construct a tissue‑damage profile that mirrored—and in many cases out‑performed—the standard clinical scores.
The Study Design and Core Findings
Patients entered the study between March 2021 and February 2023, each undergoing allogeneic HCT for blood cancers or marrow failure syndromes. Blood draws were taken at baseline, weekly for the first month, and then monthly up to six months post‑transplant. The team used bisulfite sequencing to decode methylation signatures, then fed the data into an integrative model that also considered total cfDNA load, monocyte/macrophage‑derived cfDNA, and serum alanine transaminase (ALT) levels.
"We were stunned to see that even asymptomatic patients showed elevated liver‑derived cfDNA," said Shaked ED. "It suggests a silent churn of tissue that our current monitoring would miss entirely."
The model achieved 86% specificity and 89% precision, with an area under the receiver‑operating‑characteristic curve (AUC) of 0.80 for identifying active cGVHD. Notably, the assay detected subclinical organ involvement in 27% of patients who otherwise appeared healthy, flagging them for pre‑emptive immunosuppression.
Related Research Expands the Promise
Parallel work published in the Proceedings of the National Academy of Sciences (PNAS) took a broader view. That team profiled cfDNA from 27 HCT recipients at 170 time points, applying low‑coverage bisulfite sequencing to a single plasma sample. Their algorithm could simultaneously predict acute GVHD, infectious viral reactivation, relapse of the underlying malignancy, and graft failure—all from one blood draw. The AUC for acute GVHD prediction was an impressive 0.88, and donor‑specific cfDNA fractions served as a reliable barometer for disease relapse.
Another strand of evidence comes from a study in Nature Communications, which focused on liver transplantation. Researchers at Georgetown, Washington, D.C. collected serial serum samples from 28 liver‑transplant patients and linked sustained elevations of hepatocyte‑derived cfDNA to biopsy‑confirmed allograft injury. In essence, the same cfDNA methylation approach works across different transplant types.

Implications for Transplant Care
From a clinician’s standpoint, the biggest win is the potential to intervene before organ damage becomes irreversible. Early‑stage cGVHD often responds to modest adjustments in immunosuppression, but once fibrosis sets in, treatment options narrow dramatically. By catching the molecular whisper early, physicians could fine‑tune therapy, potentially reducing the need for high‑dose steroids and their dreaded side effects.
Insurance companies are also watching. A single assay that replaces multiple biopsies, imaging studies, and lab panels could slash costs—especially if it prevents expensive hospitalizations for severe GVHD.
Future Directions and Remaining Hurdles
While the data are exciting, the path to routine clinical use isn’t flat. Larger, multi‑center trials are needed to verify performance across diverse patient populations and transplant protocols. Moreover, standardizing cfDNA extraction and sequencing pipelines will be crucial for reproducibility.
Regulatory approval is on the horizon. The study was funded by the Ernest and Bonnie Beutler Research Program of Excellence in Genomic Medicine, the Israel Science Foundation, the Waldholtz/Pakula family, the Robert M. and Marilyn Sternberg Family Charitable Foundation, and the Helmsley Charitable Trust. Their backing signals confidence that the technology will move from bench to bedside within the next few years.
In the meantime, transplant centers are already piloting cfDNA monitoring as part of post‑transplant surveillance protocols. If the early data hold, patients could soon get a simple blood draw at each clinic visit and receive a personalized risk score for cGVHD, infection, relapse, and graft health—all in one report.
Frequently Asked Questions
How does cfDNA testing improve detection of chronic GVHD compared to current methods?
Traditional monitoring relies on physical exams, liver enzyme tests, and sometimes invasive biopsies, which can miss early tissue damage. cfDNA methylation pinpoints organ‑specific injury at the molecular level, flagging subclinical disease weeks before symptoms appear, allowing clinicians to adjust therapy sooner.
Which organs can be monitored with the cfDNA methylation panel?
The panel used in the 2023 study tracks DNA fragments from the liver, skin, lungs, colon, and immune cells such as monocytes/macrophages. Additional research has shown it can be expanded to include heart, kidney, and even biliary epithelium in liver‑transplant patients.
What is the accuracy of the cfDNA assay for predicting GVHD?
In the primary cohort of 101 HCT recipients, the integrated model achieved 86% specificity and 89% precision, with an AUC of 0.80 for detecting chronic GVHD. A related PNAS study reported an AUC of 0.88 for acute GVHD prediction using a similar approach.
Will cfDNA testing replace biopsies altogether?
Not immediately. While cfDNA offers a powerful non‑invasive screen, biopsies still provide definitive histology, especially when treatment decisions are high‑risk. Over time, cfDNA could reduce the number of required biopsies by ruling out disease in low‑risk patients.
When might patients see cfDNA testing available in routine transplant follow‑up?
If ongoing multi‑center trials confirm the early results, regulatory clearance could arrive within the next two to three years. Some leading transplant centers are already enrolling patients in pilot programs, so early adopters may have access sooner.